Goals :
introduce students to the works of V. M. Garshin, introduce them to the artistic world of the writer; help students understand the moral problems of the fairy tale “Attalea princeps”; to form moral guidelines in children.
During the classes
I. Organizational stage. Creating an emotional mood.
II. The teacher's story about the life and work of the writer.
The teacher's story can be replaced by a student's message or work with a textbook article.
III. Teacher reading a fairy tale.
IV.“Attalea princeps”: heroic and ordinary in a fairy tale. Antithesis as the main artistic device. Pathos of the work * .
1. Conversation on questions:
– What is this fairy tale about?
– Is there anything in common between this work and A. Pogorelsky’s fairy tale “The Black Hen”?
– How do you imagine the characters?
2. Vocabulary work.
џ Find explanations of the words “pride” and “arrogance” in the explanatory dictionary. Write them down in your notebook. About which of the heroes of the Russian fairy tales you have read (“The Magic Ring”, “The Scarlet Flower”, “Finist – Yasnyi Sokol”) we can say:
- “This is a proud man” - “this man is eaten up by pride.”
џ Write down one description of a proud, arrogant, arrogant person according to the following plan:
- height;
– head position (head raised, shoulders straight; head raised);
– facial expression (nose, lips, eyes);
– hairstyle;
– gait;
- cloth.
џ Play out the role of a proud and proud person at home in front of your parents: how he speaks, how he walks. Then one of you will have to act out these roles in class.
3. Analysis of the tale by episode.
A) Analysis of the 1st fragment of a fairy tale from the words: “In one big city...” – to the words: “... there the leaves turned pale, shrank and withered.”
(Important points that are worth focusing on: the intonations at the beginning of the tale are calm, narrative. The first paragraph is admiring the greenhouse as a wonderful work of art, the creation of human hands. In the second paragraph, along with the words “prisoned plants,” anxiety appears, the intonations become tense.)
– From what sides does the writer show us the greenhouse? What is its duality? (On the one hand, this is a beautiful “gem”; on the other hand, it is a prison for plants.)
How did the plants feel in the greenhouse? How do you understand the phrase: “No matter how transparent a glass roof is, it is not a clear sky”?
– What was the air like in the greenhouse? What kind of wind did the plants dream about?
– What attitude does the reader have towards the greenhouse after the first page of the fairy tale?
b) Analysis of the 2nd fragment of the tale from the words: “But the glass was installed very quickly” - to the words: “...and the next day he was already on the boat home.”
When describing the director and reading his remarks, a dry, pedantic tone sounds, somewhat grumpy and hostile. When reading the Brazilian’s remarks and the paragraph dedicated to his memories of his homeland, the tone becomes lyrical, dreamy, and thoughtful.
– Do you think the director of the greenhouse and the traveler from Brazil are similar to each other? How are they different?
– What is the main occupation of the director? What has the Brazilian been doing in recent years? (The director and the Brazilian are opposed to each other: the first sits in a glass booth and examines drugs, the second travels around the world and sees the whole world. The first sees and hears only what he wants to see and hear (refuses to find out the native name of the palm tree); the eyes of the second are open to the world , he perceives phenomena in their entirety, without rejecting the opinions of other people (“... I fully believe you that botanists call it Attalea, but it also has a native, real name.”)
– Why did the Brazilian decide to leave for his homeland?
– Which person – a director or a traveler – is closer in spirit to a palm tree?
V) Analysis of the 3rd fragment of the tale from the words: “But the palm tree remained” - to the words: “The rest, although they were silent, were still angry with Attalea for her words.”
The first paragraph of this passage shows us the beautiful soul of the palm tree, its experiences and longing. She is contrasted with plants chattering among themselves: a capricious sago palm, a smug cactus, a philistine cinnamon satisfied with life, a sarcastic fern. The dispute between the plants is interrupted by a proud palm tree, calling them to break free together.
– What is the behavior of plants like in a greenhouse? What did they care about? What were they proud of?
– Why did the plants begin to prove to the palm tree that it was offering them “terrible nonsense”? What is the reason for their outrage? (Fear for your life, fear of change.)
– Why didn’t the plants support the palm tree in its quest for freedom? Why were they hostile to the palm tree, wishing it harm, like the sago palm, and being angry with it?
G) Analysis of the 4th fragment of the fairy tale from the words: “Only one little grass...” – to the words: “... sometimes remember your little friend!” (pp. 157–159).
By changing the intonation and volume of the voice when reading a fairy tale, one must try to convey the contrast between the palm tree and the little grass and at the same time the love and respect that the grass felt for the palm tree.
– Why did the grass, unlike other plants, understand the palm tree? (“She did not know southern nature, but she also loved air and freedom. The greenhouse was a prison for her too.”)
– Why did the grass want the palm tree to come into the light of God? Why did you think that you would never achieve freedom yourself?
– How does weed make us feel? (We feel sorry for her and admire her ability to sympathize and understand the feelings of the palm tree.)
d) Analysis of the 5th fragment of the fairy tale from the words: “Then the palm tree began to grow” – to the words: “The straightened green crown of a palm tree proudly rose above the glass vault.”
This is the most dynamic passage. During reading, intonations become more and more intense, and the anticipation of the outcome causes excitement. The plants are surprised, but still try to gloat. The grass sympathizes and takes pity on the palm tree. Before us is the culmination of the fairy tale. The tension of struggle is replaced by the proud intonations of victory.
– How did the director of the botanical garden feel when looking at the rapidly growing palm tree?
– Why was the palm tree ready to let out a cry of anger when struck with a cane?
– How did the palm tree fight for freedom? What price did she pay for the desire to see the real sky? (“Then the trunk began to bend. Its leafy top was crumpled, the cold rods of the frame dug into the tender young leaves, cut and mutilated them, but the tree was stubborn, did not spare the leaves, no matter what, it pressed on the bars, and the bars were already giving in, although were made of strong iron.")
Why did the little grass freeze with excitement?
- Why didn’t the palm tree want pity? Why did she say: “I will die or I will be free!”?
– What ideal did the palm tree strive for?
– What feelings do you think the director experienced when he saw the proudly straightened top of the palm tree?
e) Analysis of the 6th fragment of the fairy tale from the words: “Only something? “she thought,” to the end.
– The palm tree achieved its goal: it broke through the roof of the greenhouse, broke free and saw the sky. Why was the palm tree disappointed? Why did she understand that it was all over for her?
– What did the palm tree expect to see and what did it actually see?
Let us emphasize the contradiction between dreams and reality, the opposition between the ideal and the acquired reality.
– How did the director reason when deciding that the palm tree should be cut down? Why did he order the little grass to be thrown away?
– What feelings do we experience when we read about how a palm tree died? Are we experiencing the death of weed?
V. Reflective stage of the lesson.
Homework: answer the question posed in the “Let’s reflect on what we read” section.
Topic: “CHEMICAL ORGANIZATION OF THE CELL.
INORGANIC SUBSTANCES"
Target: 1. To introduce the chemical composition of cells, the structure of water, mineral salts and their importance for the life of the cell, to teach how to prove the material unity of the world based on knowledge about the elementary composition of cells.
2.Develop speech, memory, thinking, attention
3. Develop collective relationships: the ability to work in parks, groups, listen to comrades, cultivate a caring attitude towards water - the source of life.
Lesson Plan
Checking homework. Methodology – TIMED PEA SEA -15 minutes
Studying a new topic – 15 minutes.
A. Teacher's explanations. 1. Elements that make up the cell. -5 minutes.
B. Work in groups. Methodology – ALL RIGHT ROUND ROBIN.-10 minutes.
2. Water, its structure and meaning
3. Mineral salts, their functions.
III. Consolidation – 10 minutes. Methodology - TIK-TEK-TOU
IV. Lesson summary: 5 minutes.
During the classes
Org. moment.
I. AOZ
Guys, in the last lesson we finished studying a big topic:
“The Development of Life on Earth and the Origin of Man,” and now our task is to see how you have mastered the material you have studied.
(repeat for a few minutes)
a) The guys work according to the method TIMED PEA SEA. In each group, shoulder partners exchange their answers to the corresponding question. P. 20, c. 1-4
1. What features are common to humans and apes?
2. What are the main races within the species Homo sapiens?
3. Can we say that at present human life has ceased to be regulated by natural selection? Do you agree with this statement, provide specific evidence.
4. Do you agree that the effect of natural selection on modern man is weakened compared to the first stages of anthropogenesis?
II. Studying a new topic – 15 minutes.
We are moving on to studying a new topic.
Lesson topic: “Chemical organization of the cell. Inorganic substances"
Accordingly, our tasks and goals of the lesson:
The teacher summarizes the goals and announces the lesson plan.
Let's move on to the first lesson plan.
1.Chemical composition of the cell
Our task is to get acquainted with the chemical elements that make up the cell.
Before starting to study this topic, I would like to put before you problematic issue: “Do the same chemical elements form living and inanimate nature?”
And throughout the lesson we will look for the answer to this question.
A. Teacher's explanations. 1. Elements that make up the cell. -5 minutes.
Of the known more than 100 chemicals. There are about 80 elements in organisms, and only about 24 are known what functions they perform in the cell.
The set of these elements is not accidental. Life originated in the waters of the World Ocean, and living organisms consist primarily of these elements, which form compounds that are easily soluble in water.
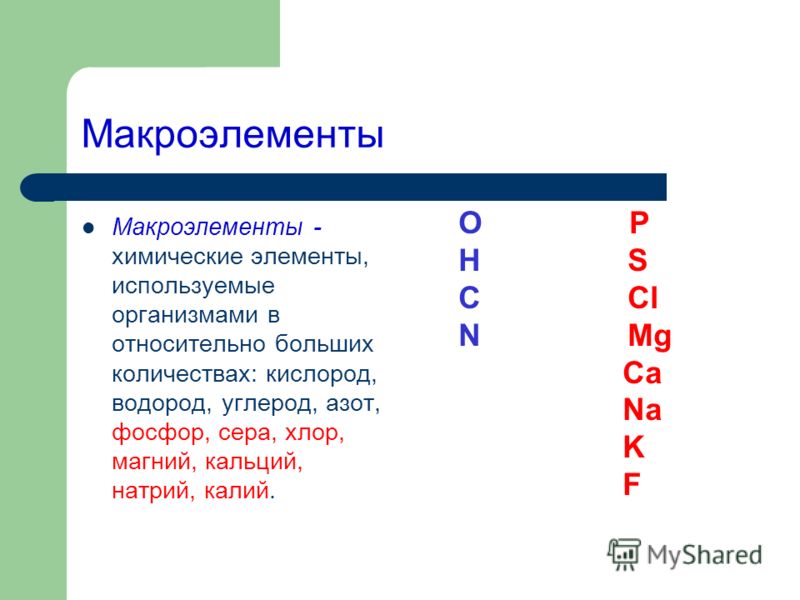
The elements that predominate in the cells of the human body are:
organogens: O 2 – 65–75%; C – 15–18%; N – 8–10%; N – 1.5–3%;
macronutrients: Mg, Na, Ca, Fe, K, S, P, Cl ≈ 4–5%;
microelements: Zn, Cu, Co, J, F, Mn ≈ 0.1%.
The cells of most animals have a similar elemental composition; Only the cells of plants and microorganisms differ.
Even those elements that are contained in cells in negligible quantities cannot be replaced by anything and are absolutely necessary for life. Thus, the iodine content in cells does not exceed 0.01%. However, if there is a lack of it in the soil (in food products), the growth and development of children is delayed.
B. Work in groups. Methodology – ALL RIGHT ROUND ROBIN.- 10 minutes.
Pupils A :
.Water, its composition and significance.
| Substance name | Elemental composition | Biological meaning |
|
| Mineral salts |
Pupils B
Mineral salts, their functions.
| Substance name | Elemental composition | Biological meaning |
|
| Mineral salts |
PHYS. JUST A MINUTE. Methodology - TAKE OFF - TOUCH DOWN.
Your task is if you agree with the statement, we stand up, if you disagree, we sit.
Life on Earth has existed for 6 thousand years. (no)
Our planet was formed approximately 4.5 billion years ago. Years ago.(yes)
The most ancient era is Archean (yes)
The first organisms lived in water. (yes)
Dinosaurs lived and died during the Mesozoic era.(Yes)
Living organisms arose by spontaneous generation from nonliving things (yes)
The first land plants - psilophytes (yes)
The first land animals were stegocephalians (yes)
Evolution can go in the opposite direction. (no)
Man appeared in the Cenozoic (yes)
Checking the completion of the table.
III. Consolidation - 10 minutes. Methodology - TIK-TEK-TOU
Guys, now each of you says one of the essential concepts from this topic:
Etc. up to 9 concepts.
We make up sentences using three words from this line.
IV. Lesson Summary
Guys, what new things did we learn in class?
Guys, why do we need this knowledge?
Where else can we use our knowledge?
Giving ratings with comments.
V. D/z Ch. 9, P.21, c. 1-4. Individual. Tasks. Describe the role of elements in the life of a cell (organism): 1. Tin and phosphorus.
2. Silver and sulfur. 3. Copper and iodine. 4. Zinc and nitrogen.
Review homework if there is time left.
Thanks for the lesson, guys.
Additional material
Beginning of the form
End of form
Horizontal: 1. This chemical element is part of the pancreatic hormone and promotes the activation of sex hormones. 2. Chemical bond, weaker than ionic bond. 3. The ability of a cell to maintain the slightly alkaline reaction of its contents at a constant level. 4. One of the cations that provides irritability in the cell.
Vertically: 5. A water molecule in which one end carries a positive charge, the other – a negative one. 6. Uneven distribution of charges in the molecule. 7.The most common inorganic compound in living organisms. 8. An essential component of vitamin B12, which is actively involved in hematopoiesis
The role of micro- and macroelements in the life of the body. Biochemical reactions are the basis of life. They provide a wide range of phenomena - from elementary mass transfer on macromolecules to the most complex mental phenomena. The quality of these reactions determines the quality of human health, his resources, ability to resist diseases, the effectiveness of recovery processes, the possibilities and degree of recovery.


MENTAL TABLE IN OUR BODY Of the 92 chemical elements found in nature, 81 are found in the human body. Of these, 12 elements are called structural, since they mainly (99%) form the elementary composition of the human body. These are carbon, oxygen, hydrogen, nitrogen, calcium, magnesium, sodium, potassium, sulfur, phosphorus, fluorine, chlorine


POTASSIUM - SODIUM: VITAL EQUILIBRIUM Potassium and sodium play a leading role in regulating the water-salt balance and acid-base balance of the body. 98% of all potassium contained in the human body is found inside cells, while 50% of all sodium is in extracellular fluid. For normal cell functioning, the ratio of the concentration of “intracellular” potassium and “extracellular” sodium is no less important


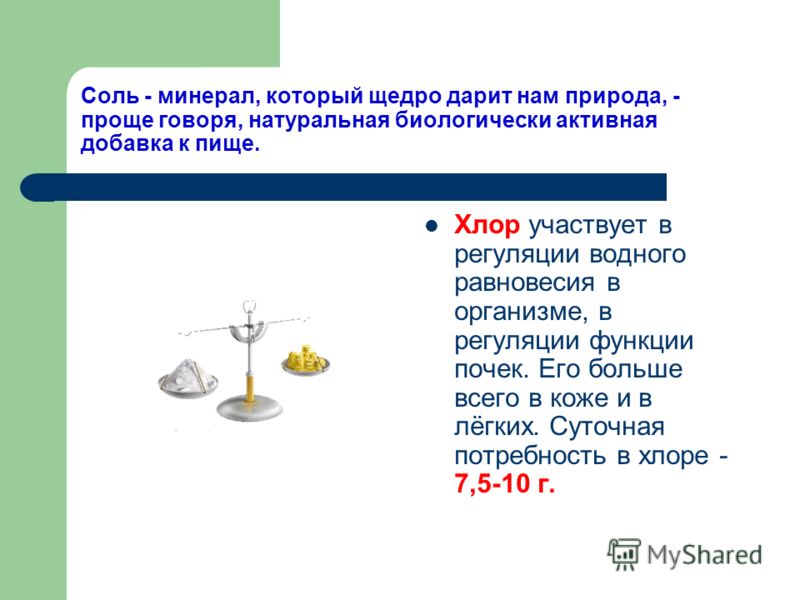
Salt is a mineral that nature generously gives us; in other words, it is a natural biologically active food additive. Chlorine is involved in the regulation of water balance in the body and in the regulation of kidney function. It is most abundant in the skin and lungs. The daily requirement for chlorine is 7.5-10 g.

Calcium (Ca) The total calcium content of the human body is approximately 1.9% of a person's total weight, with 99% of the total calcium occurring in the skeleton and only 1% in other body tissues and fluids. The daily calcium requirement for an adult is 0.45-0.8-1.2 g per day.

The magnesium content in the human body is approximately 21 g. The main “depot” of magnesium is in bones and muscles: bones contain magnesium phosphate 1.5%, tooth enamel - 0.75% (in carious teeth - 0.83-1.88% ). The daily requirement for magnesium is 0.250-0.350 g. Magnesium is an essential component of all cells and tissues and is involved in maintaining the ionic balance of body fluids; is part of enzymes and participates in the process of neuromuscular excitability.

About 80% of the phosphorus contained in the body is found in bone tissue. Phosphorus is involved in the metabolic processes of carbohydrates and fats, the formation of nucleic acids and proteins, and affects the functions of the nervous system. The metabolism of phosphorus in the body is closely related to the metabolism of calcium, vitamins D, B1, B6. With insufficient intake of phosphorus in children, the growth and development of bone tissue slows down, and metabolic processes in the body are disrupted.



Iron (Fe) - the total iron content in the human body is about 4.25 g. Of this amount, 57% is in blood hemoglobin, 23% in tissues and tissue enzymes, and the remaining 20% is deposited in the liver, spleen, bone marrow and represent a “physiological reserve” of iron

Zinc The daily human requirement for zinc is mg. Helps pancreatic cells produce insulin. Participates in fat, protein and vitamin metabolism, the synthesis of a number of hormones. stimulates general immunity and resistance to infections.



Tasks Task 1. One glass of whole milk contains 288 mg of calcium. How much milk do you need to drink per day to supply your body with enough of this element? Task 2. I always eat calcium and magnesium - there are no health problems! What in this sentence might be objectionable from a chemist's point of view?

Ponomareva Elena Alexandrovna
Place of work, position:
Municipal Educational Institution Gymnasium 9 of Voronezh, chemistry teacher
Voronezh region
Characteristics of the lesson (lesson)
The level of education:
Secondary (complete) general education
The target audience:
Teacher (teacher)
Class(es):
Item(s):
The purpose of the lesson:
Updating knowledge about the molecular level of life organization. Helping students overcome difficulties that arose when completing a test on the topic “Carbohydrates.”
Tasks: Educational:
Educational:
Educators:
Lesson type:
Combined lesson
Used textbooks and teaching aids:
Gabrielyan O.S. Chemistry. Grade 10. Basic level: textbook. for educational institutions. - M.: Bustard, 2008. - 191 p.
Methodological literature used:
Gabrielyan O.S., Yashukova A.V. Chemistry. Grade 10. Toolkit. A basic level of. - M.: Bustard, 2008. - 224 p.
Equipment used:
Computer, projector, paper handouts.
Used DSOs:
Multimedia presentations “Analysis of test work” and “Unity of the chemical organization of living organisms.”
Short description:
Lesson according to the O.S. program Gabrielyan (BUP 1 hour), 10th grade, implements the idea of natural science integration of biology, ecology, chemistry and is an introductory lesson to the topics “Oxygen-containing and nitrogen-containing substances”
Chemistry lesson
Unity of the chemical organization of living organisms
Explanatory note
Lesson according to the O.S. program Gabrielyan (BUP 1 hour) is intended for students in the 10th grade and implements the idea of natural science integration of biology, ecology, chemistry and generalization of knowledge on a chemical basis. The basis for using integration is to take into account the calendar and thematic planning of the biology course, where in basic-level classes the chemical composition of the cell (topics: “Lipids”, “Carbohydrates”, “Proteins”) is studied in October - November of the 10th grade. In addition, familiarization with the main classes of organic substances in chemistry lessons in the 9th grade, from which students know fats, proteins, and carbohydrates, makes it possible to include broader chemical knowledge than provided by the textbook, and consider the lesson as an introductory one not only to the topic “Oxygen-containing organic substances” ”, but also the subsequent ones: “Nitrogen-containing organic substances”, “Biologically active organic compounds”.
Lesson location: The lesson is conducted after studying the topic “Hydrocarbons and their natural sources” and is intended as an introductory lesson for the next block of topics: “Oxygen-containing compounds and their occurrence in living nature”, “Nitrogen-containing compounds and their occurrence in living nature”, “Biologically active organic compounds” . Since in the previous lesson the students completed the test, time was allotted to work on the mistakes made by the students when completing the test.
Goals: Updating knowledge about the molecular level of life organization. Helping students overcome difficulties that arose when completing a test on the topic “Carbohydrates.”
Tasks: Educational: reduce the number of errors made by students when completing assignments on the topic “Hydrocarbons”; fill gaps in knowledge of the basic concepts of the topic “Hydrocarbons”; show the relationship between biological and chemical knowledge; expand students' knowledge about the role of organic substances in living nature.
Educational: to form the communicative competence of students, the ability to work in groups. Develop the ability to analyze information, compare, draw conclusions, and work with additional sources.
Educators: to form a holistic natural science picture of the world; to cultivate a common personal culture.
Means of education: computer, projector, multimedia presentations “Analysis of test work” and “Unity of the chemical organization of living organisms”, handouts on paper.
During the classes
1. Organizational moment(1 minute). Communicate lesson objectives. Creating a positive emotional atmosphere.
2. Analysis of typical errors admitted by students in the test (15-20 minutes).
Included test tasks with a choice of answers and with a short open answer. Therefore, it is advisable to give students the opportunity to see their mistakes themselves, checking their answer with the correct one, and, if necessary, ask questions. The presentation combines questions and the correct answers to them, which allows not only the students who completed this option, but the whole class to work on them. Slides 2 to 13 are intended for this. The teacher addresses the remaining slides (15 - 33) only in that case, if students have a question. The transition to a more detailed commentary is carried out via a hyperlink, which allows you to rationally use time at this stage of the lesson.
Note: Question A6 in all variants involved the choice of formulas for substances that enter into a substitution reaction. In accordance with the program, the selection of saturated and/or aromatic hydrocarbons was assumed. However, if there are students in the class who are more deeply interested in chemistry and plan to take the unified state exam in this subject, it is advisable to draw their attention to the possibility of substitution reactions in alkynes for the hydrogen atom associated with the sp-hybrid carbon atom, since this material is included in the Required minimum educational content in 1998, on the basis of which the test and measurement materials of the Unified State Exam are compiled. In the presentation, the transition to this comment is marked with an asterisk. In this case, it is necessary to use the commentary from the version that was performed by students with a higher level of knowledge in chemistry.
3. Learning new material.
Teacher. We talked about the fact that at the turn of the 9th-10th centuries, the Arab alchemist Abu Bakr ar-Razi first divided all chemical substances according to their origin into three kingdoms: mineral, plant and animal substances. This unique classification lasted for almost a thousand years. But at the beginning of the 19th century, the study of substances of plant and animal origin was combined into one science. Today we have to understand what was the basis for such a union, and find out how organic substances participate in the creation of the phenomenon of life.
Now we will form groups, each group will receive a task and will complete it within 5 minutes. Then we will discuss the groups' findings. Groups are created like this: the first desk is combined with the second, the third with the fourth (students sitting at the first desk turn to the second, etc.).
Note: The questions are given in somewhat excessive quantities so that there are no difficulties when forming groups and loss of time for transferring students. If the number of students in the class is smaller, you can not consider questions 3 and 8, the removal of which does not violate the logic of presenting the material.
Working with handouts(5-7 minutes).
Task 1 group. Study the text “Levels of Organization of Living Nature.” Explain what is meant by the chemical organization of living organisms. Are there similarities and differences in the chemical organization of living and nonliving nature? What is it? Why do you think all living organisms are composed of substances of the same classes?
Levels of organization of living nature
The following levels of organization of living nature are distinguished: molecular, cellular, organismal, population-species, biogeocenotic, biosphere.
Molecular level. No matter how complex or simple the structure of any living organism, they all consist of the same chemical elements and the same molecular compounds. Up to 70 chemical elements have been found in plant and animal organisms, and approximately the same number are common in the mineral world. But in inanimate nature, substances with atomic and ionic crystal lattices predominate. In living nature - molecular - nucleic acids, proteins, carbohydrates and others. At the molecular level, various vital processes of living organisms occur: metabolism, energy conversion. At this level, the transfer of hereditary information occurs, individual organelles are formed, and other processes occur. The structure of synthesized macromolecules has species and individual specificity.
Cellular level. The structural and functional unity of all living organisms is the cell. Individual organelles within the cell perform a specific function. The functions of organelles are interconnected and perform common vital processes.
Organismic level. In each individual organism, all life processes characteristic of all living organisms occur - nutrition, respiration, metabolism, irritability, reproduction, etc. An independent organism leaves offspring. Only an integral system of organs that specifically perform various functions forms a separate independent organism.
Population-species level. A collection of individuals of one species or group that exists for a long time in a certain part of the range, relatively separately from other populations of the same species, constitutes a population.
Biogeocenotic level. A collection of organisms of different species and varying complexity of organization, adapted to the same conditions of the natural environment, is called a biogeocenosis, or natural community. Biogeocenosis includes inorganic, organic compounds and living organisms. There is an exchange of substances and energy between the components of the biogeocenosis.
Biosphere level. The totality of all living organisms on our planet and their common natural habitat constitutes the biosphere level. At the biosphere level, the circulation of matter and energy occurs on Earth with the participation of all living organisms of the biosphere.
The organization of wildlife includes several levels. The lowest - molecular - considers the molecules of substances that make up living organisms. Substances of living and inanimate nature consist of the same chemical elements. But the substances themselves of the mineral world and living nature are different. At the same time, living organisms - from the simplest to the most complex - consist of the same substances. This unity of chemical composition is ensured by the biogeocenotic level at which the exchange of substances between individuals occurs (for example, food chains).
Task 2 group. Having studied the content of chemical elements in soil and living organisms (Table 1), find out the differences in the elemental composition of living and nonliving nature. Offer your explanation of the reasons for this difference. Which elements, in your opinion, should be classified as macroelements (think about what this word might mean), and which should be classified as microelements?
Table 1.
|
Chemical element |
In soil, % |
In living organisms, % |
|
oxygen |
||
|
aluminum |
||
|
manganese |
||
Expected student response. The composition of living and inanimate nature includes the same chemical elements. However, their quantitative content differs. In living organisms, 98% of their chemical composition is made up of four elements - carbon, oxygen, nitrogen, hydrogen. Living organisms contain almost 10 times more carbon, two times more oxygen, and three times more nitrogen. At the same time, elements that are very common in nature - Al, Si, Ti - are contained in organisms in very small quantities. This is explained by the form of substances in which elements are found in living and inanimate nature. The elements carbon, hydrogen, oxygen, and nitrogen participate in the formation of complex organic molecules that make up living organisms. The composition of inanimate nature consists mostly of minerals; one of the main components is silicon oxide (therefore, there is 220 times more silicon in inanimate nature!). Macroelements are those chemical elements that are found in cells in large quantities. These are C, H, O, N, and can also include Mg, K, Ca, Na, P, S. Microelements are those whose content in organisms is low: Fe, Al, Na, Mn, etc.
Task for group 3. Read an excerpt from the Wikipedia article on Vitalism. Do you think there is a cause-and-effect relationship between two theories: the unity of the chemical organization of living organisms and vitalism? If the theory of vitalism turned out to be false, then what other reasons could you offer to explain the unity of the molecular composition of living organisms?
Vitalism
In the history of chemistry, vitalism played a leading role in distinguishing organic and inorganic substances, following the Aristotelian distinction between the mineral kingdom and the animal and plant kingdoms. Matter was assumed to exist in two completely different forms. The main idea was the possession of organic substances, as opposed to inorganic ones, by a mystical “life force”. From this it followed and was predicted that organic compounds cannot be synthesized from inorganic ones. Even after Friedrich Wöhler synthesized urea from inorganic components in 1828, the greatest minds of the time continued to explore vitalism. At the beginning of the 19th century, Jons Jakob Berzelius, known as one of the fathers of modern chemistry, rejected the mystical explanations of vitalism, but, nevertheless, there was debate about the existence of a regulating force within living matter that maintains its functions. Louis Pasteur, shortly after his famous refutation of the theory of spontaneous generation, carried out several experiments that he felt supported the theory of vitalism. Pasteur studied fermentation processes and in 1858 showed that fermentation occurs only in the presence of living cells and in the absence of oxygen. This led him to describe fermentation as "life without air." Pasteur concluded that fermentation is a “vital action.” He found no confirmation of the claims of Berzelius, Liebig, Traube and others that fermentation occurs under the influence of chemical agents or catalysts inside cells.
Expected student response. Vitalism is a theory that considers the origin of all organic substances as the result of the action of a certain vital force. One of the reasons for the emergence of the theory was the difference that thinkers realized between the substances of the mineral and living world and the unity of the chemical organization of all living beings, which delighted scientific minds. Therefore, it was logical to assume that organic substances are synthesized with the help of some mystical regulating force inside living matter. The theory remained dominant in the first half of the 19th century and hampered the development of organic chemistry. In our opinion, the unity of chemical composition is due to the exchange of substances between the body and the environment and between living beings. In addition, the unity of chemical organization can be considered confirmation of the theory of evolution.
Task for group 4. How is the transition from the inorganic world to the organic one accomplished? Read an article about photosynthesis, a phenomenon that you studied in detail in your biology course. Write an equation for the reaction of photosynthesis and an equation for the reaction of converting glucose into starch, taking into account that the formula of starch is (C 6 H 10 O 5) n. Prepare a short story about photosynthesis.
Photosynthesis is the conversion of light energy into the energy of chemical bonds
Unlike humans and animals, all green plants and some bacteria are capable of synthesizing organic substances from inorganic compounds. This type of metabolism is called autotrophic (Greek. autos himself + trophe food). Depending on the type of energy used by autotrophs for the synthesis of organic molecules, they are divided into phototrophs and chemotrophs. Phototrophs use the energy of sunlight, and chemotrophs use chemical energy released during the oxidation of various inorganic compounds.
Green plants are phototrophs. Their chloroplasts contain chlorophyll, which allows plants to carry out photosynthesis - the conversion of sunlight energy into the energy of chemical bonds of synthesized organic compounds. From the entire spectrum of solar radiation, chlorophyll molecules absorb the red and blue parts, and the green component reaches the retina of our eyes. That's why we see most plants green.
To carry out photosynthesis, plants absorb carbon dioxide from the atmosphere, and from water bodies and soil - water, inorganic salts of nitrogen and phosphorus.
But everyone knows that when carbon dioxide and water are mixed, glucose is not formed. Photosynthesis is a complex multi-step process that requires not only sunlight and chlorophyll, but also a number of enzymes, ATP energy and carrier molecules. There are two phases of photosynthesis - light and dark.
In the light phase, photons, transferring their energy to the chlorophyll molecule, transfer the molecule to an excited state: its electrons are now easily detached. Carrier molecules grab them and move them to the other side of the membrane. Chlorophyll molecules replenish the loss of electrons by stripping them from water molecules. As a result, water is split into protons and molecular oxygen:
2H 2 O - 4e - = 4H+ + O 2
Molecular oxygen is released into the atmosphere. Protons are unable to penetrate the membrane and remain inside.
Thus, electrons delivered by carrier molecules from excited chlorophyll molecules accumulate outside the membrane, and protons formed as a result of the decomposition of water accumulate inside. A potential difference arises. When it reaches a critical value, protons, under the influence of an electric field, squeeze through the tubule of the synthetase enzyme, expending energy on the synthesis of ATP. By combining with electrons on the other side of the membrane, protons form atomic hydrogen.
Further reactions can occur in the dark, which is why it is called the dark phase. They involve atomic hydrogen, ATP and enzymes. As a result, glucose is synthesized.
In addition to glucose, the formation of saturated acids, amino acids, etc. is possible. Glucose and saturated acids are further transported to leukoplasts, where reserve nutrients are formed from them - starch and fats.
Every year, the planet's vegetation provides 200 billion tons of oxygen and 150 billion tons of organic compounds necessary for humans and animals.
Expected student response. The synthesis of organic substances from inorganic ones is carried out by green plants and some bacteria in the processes of photosynthesis or chemosynthesis. The process of photosynthesis occurs in chloroplasts in the presence of the green leaf pigment - chlorophyll - with the participation of a number of enzymes. This uses light energy. The overall equation for photosynthesis is:
chlorophyll, light
6 CO 2 + 6 H 2 O ---------------> C 6 H 12 O 6 + 6 O 2
enzymes
Glucose is converted to starch according to the equation: nC 6 H 12 O 6 ---------> (C 6 H 10 O 5) n + nH 2 O
Task 5 group. Explain what the genetic code is. What chemical molecules provide the coding of genetic information? What does the property of code mean - universality? How do biologists explain the presence of this property?
Modern biology claims that one of the main features of life is self-reproduction. Genetic information is recorded in the chain of a DNA molecule.
The structure of the DNA molecule was studied in 1953 by J. Watson and F. Crick. They found that the DNA molecule consists of two chains that form a double twisted helix. Nucleotide residues are “attached” to the polymer backbone of the DNA helical chain (consists of alternating phosphate and deoxyribose carbohydrate residues). Hydrogen bonds occur between the purine base of one chain and the pyrimidine base of another chain. These bases make up complementary pairs (from the Latin complementum - addition). The formation of hydrogen bonds between complementary base pairs is due to their spatial correspondence. Each human cell contains 46 DNA molecules, which are distributed in 23 pairs of chromosomes. Chromosomes are structures along which a complete DNA molecule is distributed. The total length of all 46 DNA molecules in one human cell is about 2 meters. The total length of all DNA molecules in the body of an adult human, consisting of 5x10 13 cells, is 10 11 km, which is a thousand times greater than the distance from the Sun to the Earth.
Genetic code. The sequence of DNA nucleotides determines the sequence of amino acids in proteins - their primary structure. DNA molecules are the templates for the synthesis of all proteins.
A piece of DNA that carries information about the primary structure of a particular protein is called a gene. The corresponding sequence of nucleotides is the genetic code of the protein.
The code is universal. The genetic code is universal for all organisms on Earth. The same amino acids are encoded by the same nucleotide triplets in bacteria and elephants, algae and frogs, turtles and horses, birds and even humans.
The unity of the genetic code serves as an argument in favor of a single evolutionary path for all life on Earth.
An error in at least one triplet leads to serious disorders in the body. In patients with sickle anemia (their red blood cells are sickle-shaped rather than disc-shaped), out of the 574 amino acids of the hemoglobin protein, one amino acid is replaced by another in two places. As a result, the protein has an altered tertiary and quaternary structure. The disrupted geometry of the active center that attaches oxygen does not allow hemoglobin to effectively cope with its task - to bind oxygen in the lungs and supply it to the body's cells.
Expected student response. The most complex organic substances are nucleic acids. DNA ensures the storage and transmission of genetic information. DNA molecules are templates for the synthesis of proteins. The sequence of nucleotides - fragments of a DNA molecule encoding a sequence of amino acids - is called the genetic code. The genetic code is universal. This means that coding occurs the same in all living beings - from a virus to a person. Biologists see this as one of the proofs of evolution.
Group assignment 6. Read the text and highlight the main functions of proteins in the body.
Squirrels
Proteins are an irreplaceable building material. All cell membranes contain a protein, the role of which is varied. The protein keratin consists of hair, nails, claws, wool, feathers, hooves, and the outer layer of skin.
Many proteins have a contractile (motor) function. For example, the proteins actin and myosin are part of the muscle fibers of higher organisms.
The role of proteins in the transport of substances is great. An example of transport proteins is hemoglobin, which carries oxygen from the lungs to other tissues and carbon dioxide from tissues to the lungs, as well as proteins homologous to it, found in all kingdoms of living organisms. In muscles, this function is taken over by another transport protein - myoglobin. Blood plasma proteins transport nutrients.
Protein can also play a storage function. These proteins include ferritin (iron reserve), ovalbumin - egg protein, casein - milk protein, zein - corn seed protein.
Hormone proteins perform a regulatory function. Hormones are biologically active substances that affect metabolism. One of the most well-known hormone proteins is insulin. It reduces blood sugar, promotes glycogen synthesis in the liver and muscles, increases the formation of fats from carbohydrates, affects phosphorus metabolism, and enriches cells with potassium. Protein hormones of the pituitary gland, an endocrine gland associated with one of the parts of the brain, have a regulatory function. It secretes growth hormone, in the absence of which dwarfism develops.
Another function of proteins is protective. On its basis, a branch of science called immunology was created. Proteins that make up blood and other biological fluids are involved in the body's defense response to both damage and attack by pathogens. They neutralize bacteria, viruses or foreign proteins. For example, the protein interferon kills influenza viruses.
Recently, proteins with receptor function have been classified as a separate group. There are sound, taste, light and other receptors. There are cellular receptors built into the membrane. One part of the receptor molecule senses a signal, which is most often a chemical substance. As a result, the conformation of another part of the molecule, which transmits the signal to other cellular components, changes. Some receptors catalyze a specific chemical reaction; others serve as ion channels that open or close when triggered by a signal; still others specifically bind intracellular messenger molecules.
Enzymes - biological catalysts - protein substances. The variety of enzymes is enormous. Even in a small bacterium there are many hundreds of them. Each enzyme catalyzes only its own reaction. For example, pepsin, an enzyme in gastric juice, breaks down food proteins. Enzymes can also work outside the body. For example, many washing powders contain substances that break down dirt stains. Instant foods contain enzymes that break down proteins.
New research allows us to identify new groups of proteins with new functions. Among them are unique substances - neuropeptides (responsible for the most important life processes: sleep, memory, pain, feelings of fear, anxiety).
Expected student response. The functions of proteins are diverse. Among them we can highlight the following: proteins - building material (hair, nails, cell membranes). Proteins provide: transport of substances in the body (blood proteins), muscle contraction (muscles are built from proteins), immunity (leukocytes), sensitivity (receptor proteins), regulation (hormones), catalysis (enzymes). They can also play a storage role (for example, egg white and milk protein).
Group assignment 7. After studying the text, list the functions of carbohydrates and fats in living organisms.
The role of carbohydrates and fats in the body of living beings
The main function of carbohydrates is to supply our body with energy. The body receives the bulk of the energy it needs through the oxidation of glucose. A long-term lack of carbohydrates in the diet leads to disruption of the metabolism of fats and proteins. Harmful products of incomplete oxidation of fats and some amino acids—ketone bodies—accumulate in the blood. A serious consequence of carbohydrate deficiency is a decrease in blood glucose levels, to which the central nervous system is especially sensitive. Weakness, drowsiness, dizziness, headaches, hunger, nausea, sweating, and trembling hands occur.
Excess glucose is converted into starch (plants) or glycogen (animals), which act as storage substances. They will be converted back into glucose as needed.
The carbohydrate cellulose forms the wall of plant cells. Chitin serves as a building material for the construction of insect shells.
Fats take second place after carbohydrates as a source of energy. When 1 g of carbohydrates is broken down, 17.6 kJ of energy is released, when 1 g of fats is broken down - 38.9 kJ. Excess fat is stored in the subcutaneous layer (animals) or in fat droplets in fruits (plants).
The fat layer surrounds the internal organs, providing their mechanical protection. The subcutaneous layer performs the function of thermal insulation. Fats are involved in the formation of some hormones and biologically active substances. In addition, fats are part of cell membranes.
Expected student response. We have identified the following functions of carbohydrates: energy (the main supplier of energy), storage (starch - plants, glycogen - animals), construction (cellulose - plants, chitin - insects). Fats: energy, protective (subcutaneous layer, fat layer around internal organs), construction (cell membranes), regulatory (participation in the formation of hormones).
Group assignment 8. Remember what optical isomerism is. If you have any difficulty, read the material in the chemistry textbook on page 20 from the words “Optical isomerism is possessed...” Read the popular science text from the children's encyclopedia. Try to explain the law of chiral purity based on knowledge of biology.
About the asymmetry of the living.
The German philosopher Immanuel Kant remarked: “What could be more like my hand or my ear than their own reflection in a mirror? And yet I cannot put the hand I see in the mirror in the place of the original.” Protein chains consist of individual amino acids, which can also be right- or left-handed. Without differing in chemical composition, they will differ from each other, like an object and its mirror image. So: the proteins of living organisms contain only left-handed amino acids! Right forms are simply harmful for earthly life. When one of the Western pharmaceutical companies released a medicine that included both right and left forms, women who used it began to have sick children.
Carbohydrates can also be right or left. In living organisms, all carbohydrates are right-handed.
|
Rice. 1. Scheme of how enzymes work |
The most important life processes can only take place in a “mirror” homogeneous environment. After all, enzymes-proteins that ensure reactions occur in living organisms are adjusted to only one of the forms. This means that life inevitably had to violate the equality of right and left.
For glucose, for example, there are 16 optical isomers. During chemical synthesis, all isomeric substances are formed simultaneously. At the same time, only glucose is synthesized in nature. This is called the law of chiral purity.
Expected student response. Most complex organic substances have optical isomers. Optical isomers are mirror images of each other. However, the law of chiral purity operates in nature. This means that only one of the isomers exists in nature. While during chemical synthesis a mixture of them is formed. In our opinion, this happens because in nature substances are synthesized with the help of biological catalysts - enzymes that are adapted only to one specific optical isomer.
Discussion of completed tasks(15-20 minutes).
Teacher. The phenomenon of life has been studied for many centuries by biologists, ecologists, chemists, and physicists. The groups will now briefly report on what they have learned about the chemical organization of living nature.
Group 1 will explain what is meant by the chemical organization of living nature and whether there are differences at this level between living and nonliving nature. (Group response). Slide 2.
Group 2 clarified the differences between living and nonliving nature at the level of chemical elements and is ready to offer their conclusions. (Group response). Slides 3-4.
Group 3 learned that the theory of the unity of the chemical composition of living nature became one of the reasons that for a long time hampered the development of organic chemistry. And he will share with us his conclusions about how this could be connected. (Group response). Slide 5.
Group 4 found out how the transition from inorganic to organic substances occurs. (Group response). Slide 6.
Organic molecules that make up living organisms have their own characteristics and perform a specific function. The first, main group of organic compounds in living organisms includes nucleic acids - DNA, RNA. The role of these substances was determined by group 5. (Group answer). Slide 7.
The second group of organic compounds in living organisms includes proteins. These compounds are so important that one of the definitions of life is: “Life is the way of existence of protein bodies.” Group 6 found out why they are so important. (Group answer). Slide 8-9.
The third group of organic compounds includes carbohydrates and fats. Their role is known to Group 7. And they will now share with us. (Group response). Slides 10-11.
Group 8 studied a unique phenomenon that distinguishes naturally occurring organic substances from their synthetic counterparts. Until now, science has not been able to give a convincing answer to the question of its causes. But we will now find out the group’s opinion. (Group response). Slide 12.
Summarizing. Word cloud. Formulate a conclusion - 1 minute.
The class is asked to make a short summary of the lesson based on key words. Slide 13.
The conclusion may sound like this: All living organisms have a similar chemical organization that distinguishes them from inanimate nature. The main substances of living nature are nucleic acids, proteins, fats and carbohydrates.
Homework:§ 9 (pp. 63-65), individual task: student’s report on the physiological effect of ethyl alcohol on the human body.
List of sources used
1. Gabrielyan O.S. Chemistry. Grade 10. Basic level: textbook. For educational institutions. - M.: Bustard, 2008. - 191 p.
2. Gabrielyan O.S., Yashukova A.V. Chemistry. Grade 10. Toolkit. A basic level of. - M.: Bustard, 2008. - 224 p.
3. Opalovsky A.A. Planet Earth through the eyes of a chemist. - M.: Nauka, 1990. - 224 p.
4. Chemistry. Tutor manual for applicants to universities / Ed. Egorova A.S. - Rostov-on-Don: ed. Phoenix, 2002. - 768 p.
Topic: “Chemical organization of the cell. Inorganic substances"
Lesson objectives:
Educational: to develop knowledge about the role of chemical elements, water, cations, anions, salts in the life of the cell. To teach how to use knowledge about the chemical composition of a cell to prove the material unity of living and inanimate nature, the unity of the organic world.
Educational: formation of skills to analyze, highlight the main thing, compare, generalize and systematize.
Educational: instill communication skills, cultivate in students an interest in learning, the desire to achieve success through a conscientious attitude to their work.
Lesson type: a lesson in learning new material.
Lesson type: lesson using a computer.
Form of work: individual, group, in pairs.
Means of education: computer, multimedia projector, educational electronic publication “Laboratory workshop Biology grades 6-11,” presentation disk, table “Water is an unusual substance,” portrait of V.I. Vernadsky, felt-tip pens, drawings for creating a project, glue, exhibition of literature on the topic .
During the classes.
Org. moment.
Learning new material.
Vinson Brown said that “the accumulation of knowledge is like the growth of a tree” and I hope that in this lesson the powerful trunk of biological knowledge of each of you will grow with a new branch of knowledge about the chemical composition of the cell, about the role of chemical elements, water, mineral salts in the life of the cell .
When considering the presence of chemical elements on Earth, three spheres of inanimate nature are usually taken into account: the atmosphere, the hydrosphere, the lithosphere and the fourth sphere - the area of existence of living organisms - the biosphere. Russian scientist V.I. Vernadsky, conducting a detailed analysis of the content of elements in the earth's crust and in living organisms, came to the conclusion that the qualitative composition of these objects is close. He assumed that in a living organism there would someday be
found all the elements of the periodic table found in nonliving
nature of the Earth. Indeed, to date, the presence of about 70 elements of the periodic table has been reliably established in the human body.
From the table “Content of some elements in the environment and in
in the human body" it is clear that in a living organism predominate
nonmetals, and in the earth's crust - metals.
Depending on their content in a living organism, chemical elements are divided into several groups.
Fragment No. 1(disc: educational electronic edition) on the screen:
Macronutrients:
A ) H, O, C, N - 98%
+ S, P- bioelements form organic compounds.
b) K, Na, Ca, Mg, Fe, Cl - near 2%
K, Na, Cl– permeability of cell membranes, conduction of nerve impulses.
P, Ca– formation of bone tissue, bone strength.
Ca- ensures blood clotting.
Fe – is part of hemoglobin
Mg- is part of chlorophyll in plants and enzymes in animals.
Microelements– content about 0.02%
Zn is part of insulin - a hormone of the pancreas, enhances the activity of the gonads.
Cu ensures tissue growth and is part of enzymes.
I is part of thyroxine, a thyroid hormone.
F is part of the enamel of teeth.
Co is part of vitamin B12
Mn ensures metabolism.
B responsible for the growth process.
Mo responsible for the use of iron and the retention of fluoride in the body.
Lack of macro- and microelements leads to various diseases. And to prevent them, you need to eat certain foods.
Calcium. After 4 main elements it ranks fifth. In an adult, up to 700 mg is excreted from bone tissue per day. calcium and the same amount is deposited again. Consequently, bone tissue, in addition to its supporting function, plays the role of a depot of calcium and phosphorus, from where the body extracts them when there is a lack of dietary intake.
For example, when atmospheric pressure drops, the body needs more calcium than usual to maintain balance. If there are no reserves of it in the blood, then it is intensively extracted from the bones. When the process goes beyond normal limits, pathology develops, more often in the elderly, and they say “oh, how my bones hurt! This is for bad weather..."
If there is a shortage calcium osteoporosis develops (softness, porosity of bones), slowing down of skeletal growth.
It is necessary to consume dairy products.
If there is a shortage magnesium muscle cramps, loss of body fluids.
Products: vegetables, beans, nuts, milk, fruits.
If there is a shortage chlorine- dry skin.
Ingredients: water, table salt.
If there is a shortage sodium– headache, poor memory, loss of appetite
Products: tomatoes, apricots, peas, table salt.
If there is a shortage potassium– arrhythmia of heart contractions, sudden death with increasing loads.
Products – bananas, dried fruits, potatoes, tomatoes, zucchini.
Phosphorus– external signs of deficiency are unknown. Contained in fish, dairy products, walnuts, buckwheat.
If there is a shortage gland anemia develops. It is necessary to eat liver, meat, green leaves of vegetables.
If there is a shortage fluoride– tooth decay. Products: fish, water.
If there is a shortage zinc– skin damage. Products – meat, seafood.
If there is a shortage iodine goiter develops. It is necessary to eat persimmons, seafood, and iodized salt.
If there is a shortage copper– cancer, liver dysfunction. Products – liver, egg yolk, whole grains.
With a lack of cobalt, pernicious anemia develops. Products - liver, animal proteins.
There is one product that combines almost all chemical elements. What do you think it is? That's right, it's honey. By eating a teaspoon of honey a day, you help your body avoid many problems.
Primary consolidation.
Fragment No. 2(disk ) on the screen.
Question 1.
Question 2.
Question 3.
Question 4.
Question 5.
Water is the most common substance in living organisms (Appendix 1).
During the presentation prepared by the students, slides are shown on the screen, which present the properties of water, functions, water content in various organs, and statements of great people about water.
3
Mineral salts.
In addition to water, among the inorganic substances that make up the cell,
you need to name salts that are ionic compounds. In an aqueous solution, they dissociate to form a metal cation and an acid residue anion.
Cells are most important for life processes
Cations: K,Na,Ca,Mg.
Anions: H2PO4, Cl ,HCO3,
The concentration of ions on the outer surface of the cell is different from their concentration on the inner surface. The outer surface of the cell membrane has a very high concentration of sodium ions, and the inner surface has a high concentration of potassium ions. As a result, a potential difference is formed between the inner and outer surfaces of the cell membrane, which causes the transmission of excitation along a nerve or muscle.
Calcium and magnesium ions are activators of many enzymes.
Its buffering properties depend on the concentration of salts inside the cell. Buffering is the ability of a cell to maintain a slightly alkaline reaction at a constant level. Buffering inside the cell is provided by anions H2PO4 and HPO4.
In extracellular fluid and blood, H2CO3 and HCO3 play the role of a buffer.
Anions of weak acids and weak alkalis bind hydrogen ions and hydroxide ions, due to which the reaction inside the cell does not change.
Hydrochloric acid creates an acidic environment in the stomach, speeding up the digestion of food proteins.
Calcium and phosphorus ions are found in bone tissue.
Mineral salts enter the body's cells from the external environment. Excess salts along with water are excreted from the body into the external environment.
Primary consolidation.
1. What inorganic substances make up the cell?
2. What percentage of water is contained on average in the human body?
3. List the properties of water.
4. Name the functions of water.
5. What is buffering?
6. What anions does it support?
7. What are the functions of potassium, sodium, and calcium cations?
Formation of abilities and skills.
The class is divided into groups to create a lesson project.
Group assignments:
Cover.
Macroelements.
Microelements.
Water.
Mineral salts.
Students defend their work after completion.
4. Lesson summary.
